Molecular Shape |
|---|
The three dimensional shape or
configuration of a molecule is an important characteristic.
This shape is dependent on the preferred spatial orientation of
covalent bonds to atoms having two or more bonding partners. Three
dimensional configurations are best viewed with the aid of models.
 In order
to represent such configurations on a two-dimensional surface
(paper, blackboard or screen), we often use perspective
drawings in which the direction of a bond is specified by the
line connecting the bonded atoms.
In order
to represent such configurations on a two-dimensional surface
(paper, blackboard or screen), we often use perspective
drawings in which the direction of a bond is specified by the
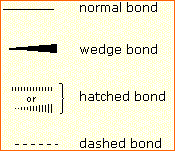
line connecting the bonded atoms. 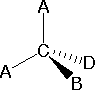 In most cases the
focus of configuration is a carbon atom so the lines specifying bond
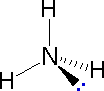
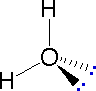
directions will originate there. As defined in the diagram on the
right, a simple straight line represents a bond lying approximately
in the surface plane. The two bonds to substituents A in the
structure on the left are of this kind. A wedge shaped bond is
directed in front of this plane (thick end toward the viewer), as
shown by the bond to substituent B; and a hatched bond is
directed in back of the plane (away from the viewer), as shown by
the bond to substituent D. Some texts and other sources may
use a dashed bond in the same manner as we have defined the hatched
bond, but this can be confusing because the dashed bond is often
used to represent a partial bond (i.e. a covalent bond that is
partially formed or partially broken). The following examples make
use of this notation, and also illustrate the importance of
including non-bonding valence shell electron pairs (colored blue)
when viewing such configurations .
In most cases the
focus of configuration is a carbon atom so the lines specifying bond
directions will originate there. As defined in the diagram on the
right, a simple straight line represents a bond lying approximately
in the surface plane. The two bonds to substituents A in the
structure on the left are of this kind. A wedge shaped bond is
directed in front of this plane (thick end toward the viewer), as
shown by the bond to substituent B; and a hatched bond is
directed in back of the plane (away from the viewer), as shown by
the bond to substituent D. Some texts and other sources may
use a dashed bond in the same manner as we have defined the hatched
bond, but this can be confusing because the dashed bond is often
used to represent a partial bond (i.e. a covalent bond that is
partially formed or partially broken). The following examples make
use of this notation, and also illustrate the importance of
including non-bonding valence shell electron pairs (colored blue)
when viewing such configurations .
 |
 |
 | ||
| Methane | Ammonia | Water |
|---|
Bonding configurations are readily predicted by valence-shell electron-pair repulsion theory, commonly referred to as VSEPR in most introductory chemistry texts. This simple model is based on the fact that electrons repel each other, and that it is reasonable to expect that the bonds and non-bonding valence electron pairs associated with a given atom will prefer to be as far apart as possible. The bonding configurations of carbon are easy to remember, since there are only three categories.
| Configuration | Bonding Partners | Bond Angles | Example |
|---|---|---|---|
| Tetrahedral | 4 | 109.5║ |
|
| Trigonal | 3 | 120║ | |
| Linear | 2 | 180║ |
In the
three examples shown above, the central atom (carbon) does not have
any non-bonding valence electrons; consequently the configuration
may be estimated from the number of bonding partners alone. For
molecules of water and ammonia, however, the non-bonding electrons
must be included in the calculation. In each case there are four
regions of electron density associated with the valence shell so
that a tetrahedral bond angle is expected. The measured bond angles
of these compounds (H2O 104.5║ &
NH3 107.3║) show that they are closer
to being tetrahedral than trigonal or linear. Of course, it is the
configuration of atoms (not electrons) that defines the the shape of
a molecule, and in this sense ammonia is said to be pyramidal (not
tetrahedral). The compound boron trifluoride, BF3, does not have non-bonding valence electrons
and the configuration of its atoms is trigonal.
One way in which
the shapes of molecules manifest themselves experimentally is
through molecular dipole moments. A molecule which has one or more
polar covalent bonds may have a dipole moment as a result of the
accumulated bond dipoles. In the case of water, we know that the O-H
covalent bond is polar, due to the different electronegativities of
hydrogen and oxygen. Since there are two O-H bonds in water, their
bond dipoles will interact and may result in a molecular dipole
which can be measured. The following diagram shows four possible
orientations of the O-H bonds.
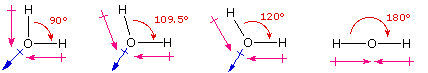
The bond
dipoles are colored magenta and the resulting molecular dipole is
colored blue. In the linear configuration (bond angle 180║) the bond
dipoles cancel, and the molecular dipole is zero. For other bond
angles (120 to 90║) the molecular dipole would vary in size, being
largest for the 90║ configuration. In a similar manner the
configurations of methane (CH4) and
carbon dioxide (CO2) may be deduced
from their zero molecular dipole moments. Since the bond dipoles
have canceled, the configurations of these molecules must be
tetrahedral (or square-planar) and linear respectively.
The case
of methane provides insight to other arguments that have been used
to confirm its tetrahedral configuration. For purposes of discussion
we shall consider three other configurations for CH4, square-planar, square-pyramidal and
triangular-pyramidal.
Substitution of one hydrogen by a chlorine atom gives
a CH3Cl compound. Since the
tetrahedral, square-planar and square-pyramidal configurations have
structurally equivalent hydrogen atoms, they would each give a
single substitution product. However, in the trigonal-pyramidal
configuration one hydrogen (the apex) is structurally different from
the other three (the pyramid base). Substitution in this case should
give two different CH3Cl compounds if
all the hydrogens react. In the case of disubstitution, the
tetrahedral configuration of methane would lead to a single
CH2Cl2
product, but the other configurations would give two different
CH2Cl2
compounds. These substitution possibilities are shown in the above
Chime insert.
Isomers |
|---|
Structural Formulas
It is necessary to draw
structural formulas for organic compounds because in most cases a
molecular formula does not uniquely represent a single compound.
Different compounds having the same molecular formula are called
isomers, and the prevalence of organic isomers reflects the
extraordinary versatility of carbon in forming strong bonds to
itself and to other elements.
When the group of atoms that make
up the molecules of different isomers are bonded together in
fundamentally different ways, we refer to such compounds as
constitutional isomers. There are seven constitutional
isomers of C4H10O, and structural formulas for these are drawn
in the following table. These formulas represent all known and
possible C4H10O compounds, and display a common structural
feature. There are no double or triple bonds and no rings in any
of these structures.
| KekulÚ Formula | Condensed Formula | Shorthand Formula |
|---|---|---|
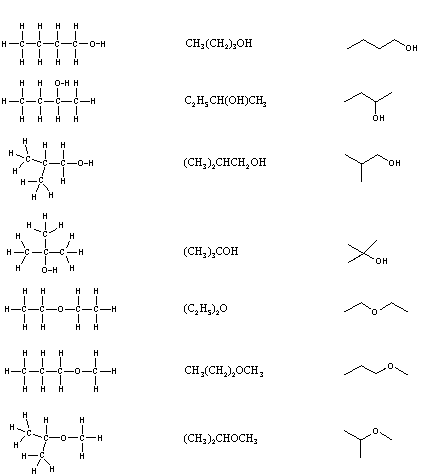 | ||
Simplification of structural formulas may be achieved
without any loss of the information they convey. In condensed
structural formulas the bonds to each carbon are omitted, but
each distinct structural unit (group) is written with subscript
numbers designating multiple substituents, including the hydrogens.
Shorthand (line) formulas omit the symbols for carbon and
hydrogen entirely. Each straight line segment represents a bond, the
ends and intersections of the lines are carbon atoms, and the
correct number of hydrogens is calculated from the tetravalency of
carbon. Non-bonding valence shell electrons are omitted in these
formulas.
Developing the ability to visualize a three-dimensional
structure from two-dimensional formulas requires practice, and in
most cases the aid of molecular models. As noted earlier, many kinds
of model kits are available to students and professional chemists,
and the beginning student is encouraged to obtain one.
Distinguishing Carbon Atoms:
When discussing
structural formulas, it is often useful to distinguish different
groups of carbon atoms by their structural characteristics. A
primary carbon (1║) is one that is bonded to no more than one
other carbon atom. A secondary carbon (2║) is bonded to two
other carbon atoms, and tertiary (3║) and quaternary
(4║) carbon atoms are bonded respectively to three and four other
carbons. The three C5H12 isomers shown below illustrate these
terms.
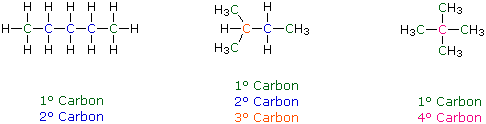
Structural differences may occur within these four groups, depending on the molecular constitution. In the formula on the right all four 1║-carbons are structurally equivalent (remember the tetrahedral configuration of tetravalent carbon); however the central formula has two equivalent 1║-carbons (bonded to the 3║ carbon on the left end) and a single, structurally different 1║-carbon (bonded to the 2║-carbon) at the right end. Similarly, the left-most formula has two structurally equivalent 2║-carbons (next to the ends of the chain), and a structurally different 2║-carbon in the middle of the chain. A consideration of molecular symmetry helps to distinguish structurally equivalent from nonequivalent atoms and groups. The ability to distinguish structural differences of this kind is an essential part of mastering organic chemistry. It will come with practice and experience.
Formula Analysis |
|---|
Although structural formulas are essential to the unique description of organic compounds, it is interesting and instructive to evaluate the information that may be obtained from a molecular formula alone. Three useful rules may be listed:
| Some Plausible Molecular Formulas |
C4H4Cl2, C5H9OBr, C5H11NO2, C12H18N2FCl |
| Some Impossible Molecular Formulas |
C5H9O2, C4H5ClBr, C6H11N2O, C10H18NCl2 |
| Some Plausible Molecular Formulas |
C7H16O3, C9H18, C15H28O3, C6H16N2 |
| Some Impossible Molecular Formulas |
C8H20O6, C23H50, C5H10Cl4, C4H12NO |
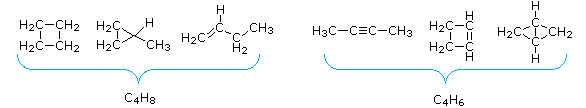 |
From the above discussion and examples it should be clear that the molecular formula of a hydrocarbon (CnHm) provides information about the number of rings and/or double bonds that must be present in its structural formula. A triple bond is counted as two double bonds.
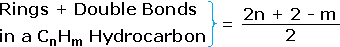
|
|---|
This formula may be extended beyond hydrocarbons by a few simple corrections:
Resonance |
|---|
KekulÚ structural formulas are essential tools for understanding organic chemistry. However, the structures of some compounds and ions cannot be represented by a single formula. For example, sulfur dioxide (SO2) and nitric acid (HNO3) may each be described by two equivalent formulas (equations 1 & 2). For clarity the two ambiguous bonds to oxygen are given different colors in these formulas.
| 1) sulfur dioxide | 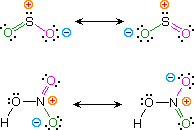 | |
| 2) nitric acid |
If only one formula for sulfur dioxide was correct and accurate, then the double bond to oxygen would be shorter and stronger than the single bond. Since experimental evidence indicates that this molecule is bent (bond angle 120║) and has equal length sulfur : oxygen bonds (1.432 ┼), a single formula is inadequate, and the actual structure resembles an average of the two formulas. This averaging of electron distribution over two or more hypothetical contributing structures (canonical forms) to produce a hybrid electronic structure is called resonance. Likewise, the structure of nitric acid is best described as a resonance hybrid of two structures, the double headed arrow being the unique symbol for resonance.
The above examples represent one extreme in the application of resonance. Here, two structurally and energetically equivalent electronic structures for a stable compound can be written, but no single structure provides an accurate or even an adequate representation of the true molecule. In cases such as these, the electron delocalization described by resonance enhances the stability of the molecules, and compounds or ions composed of such molecules often show exceptional stability.
| 3) formaldehyde | 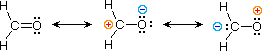 |
The electronic structures of most covalent compounds do not suffer the inadequacy noted above. Thus, completely satisfactory KekulÚ formulas may be drawn for water (H2O), methane (CH4) and acetylene C2H2). Nevertheless, the principles of resonance are very useful in rationalizing the chemical behavior of many such compounds. For example, the carbonyl group of formaldehyde (the carbon-oxygen double bond) reacts readily to give addition products. The course of these reactions can be explained by a small contribution of a dipolar resonance contributor, as shown in equation 3. Here, the first contributor (on the left) is clearly the best representation of this molecular unit, since there is no charge separation and both the carbon and oxygen atoms have achieved valence shell neon-like configurations by covalent electron sharing. If the double bond is broken heterolytically, formal charge pairs result, as shown in the other two structures. The preferred charge distribution will have the positive charge on the less electronegative atom (carbon) and the negative charge on the more electronegative atom (oxygen). Therefore the middle formula represents a more reasonable and stable structure than the one on the right. The application of resonance to this case requires a weighted averaging of these canonical structures. The double bonded structure is regarded as the major contributor, the middle structure a minor contributor and the right hand structure a non-contributor. Since the middle, charge-separated contributor has an electron deficient carbon atom, this explains the tendency of electron donors (nucleophiles) to bond at this site.
The basic principles of the resonance method may
now be summarized.
For a given compound, a set of Lewis /
KekulÚ structures are written, keeping the relative positions of all
the component atoms the same. These are the canonical forms to be
considered, and all must have the same number of paired and unpaired
electrons.
The following factors are important in evaluating
the contribution each of these canonical structures makes to the
actual molecule.
The stability of a resonance hybrid is always greater than the stability of any canonical contributor. Consequently, if one canonical form has a much greater stability than all others, the hybrid will closely resemble it electronically and energetically. This is the case for the carbonyl group (eq.3). The left hand C=O structure has much greater total bonding than either charge-separated structure, so it describes this functional group rather well. On the other hand, if two or more canonical forms have identical low energy structures, the resonance hybrid will have exceptional stabilization and unique properties. This is the case for sulfur dioxide (eq.1) and nitric acid (eq.2).
| 4) carbon monoxide | 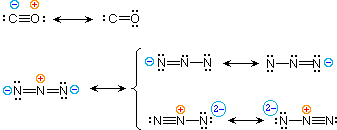 | |
| 5) azide anion |
To illustrate these principles we shall consider carbon monoxide (eq.4) and azide anion (eq.5). In each case the most stable canonical form is on the left. For carbon monoxide, the additional bonding is more important than charge separation. Furthermore, the double bonded structure has an electron deficient carbon atom (valence shell sextet). A similar destabilizing factor is present in the two azide canonical forms on the top row of the bracket (three bonds vs. four bonds in the left most structure). The bottom row pair of structures have four bonds, but are destabilized by the high charge density on a single nitrogen atom.
All the examples on this page demonstrate an important restriction that must be remembered when using resonance. No atoms change their positions within the common structural framework. Only electrons are moved.
Orbitals |
|---|
A more detailed model of covalent bonding requires a consideration of valence shell atomic orbitals. For second periob elements such as carbon, nitrogen and oxygen, these orbitals have been designated 2s, 2px, 2py & 2pz. The spatial distribution of electrons occupying each of these orbitals is shown in the diagram below. To use an elegant orbital visualization resource, created at MIT, Click Here
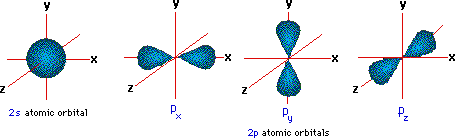
The valence shell electron configuration of carbon is 2s2, 2px1, 2py1 & 2pz0. If this were the configuration used in covalent bonding, carbon would only be able to form two bonds.
Hybrid Orbitals
In order to explain the
structure of methane (CH4), the 2s
and three 2p orbitals must be converted to four equivalent hybrid
atomic orbitals, each having 25% s and 75% p character, and
designated sp3. These hybrid orbitals
have a specific orientation, and the four are naturally oriented in
a tetrahedral fashion.
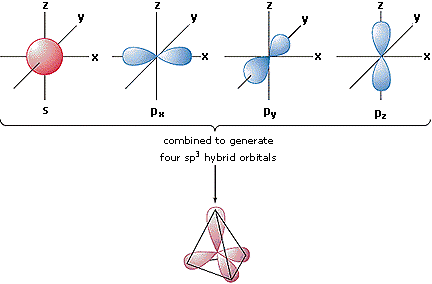
Molecular Orbitals
Just as the valence
electrons of atoms occupy atomic orbitals (AO), the shared electron
pairs of covalently bonded atoms may be thought of as occupying
molecular orbitals (MO). It is convenient to approximate molecular
orbitals by combining or mixing two or more atomic orbitals. In
general, this mixing of n atomic orbitals always generates
n molecular orbitals. The hydrogen molecule provides a simple
example of MO formation. In the following diagram, two 1s atomic
orbitals combine to give a sigma (σ) bonding (low energy) molecular
orbital and a second higher energy MO referred to as an antibonding
orbital. The bonding MO is occupied by two electrons of opposite
spin, the result being a covalent bond.
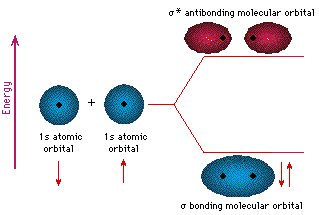
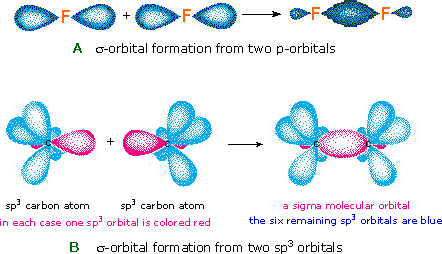
The notation used for molecular orbitals parallels that used for atomic orbitals. Thus, s-orbitals have a spherical symmetry surrounding a single nucleus, whereas σ-orbitals have a cylindrical symmetry and encompass two (or more) nuclei. In the case of bonds between second period elements, p-orbitals or hybrid atomic orbitals having p-orbital character are used to form molecular orbitals. For example, the sigma molecular orbital that serves to bond two fluorine atoms together is generated by the overlap of p-orbitals (part A below), and two sp3 hybrid orbitals of carbon may combine to give a similar sigma orbital. When these bonding orbitals are occupied by a pair of electrons a covalent bond, the sigma bond results
Another type of MO (the π orbital) may be formed from two p-orbitals by a lateral overlap, as shown in part A of the following diagram. Since bonds consisting of occupied π-orbitals (pi-bonds) are weaker than sigma bonds, pi-bonding between two atoms occurs only when a sigma bond has already been established. Thus, pi-bonding is generally found only as a component of double and triple covalent bonds. Since carbon atoms involved in double bonds have only three bonding partners, they require only three hybrid orbitals to contribute to three sigma bonds. A mixing of the 2s-orbital with two of the 2p orbitals gives three sp2 hybrid orbitals, leaving one of the p-orbitals unused. Two sp2 hybridized carbon atoms are then joined together by sigma and pi-bonds (a double bond), as shown in part B.
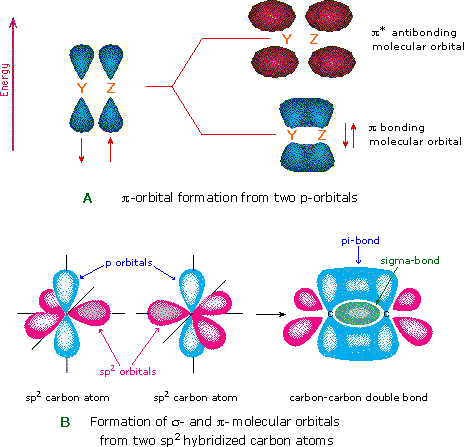
The p-orbitals in this model are represented by red and blue colored spheres, which represent different phases, defined by the mathematical wave equations for such orbitals.
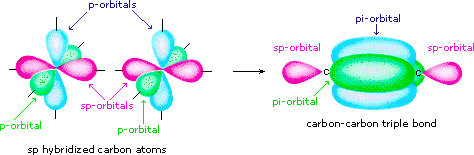
Finally, in the case of carbon atoms with only two bonding partners only two hybrid orbitals are needed for the sigma bonds, and these sp hybrid orbitals are directed 180║ from each other. Two p-orbitals remain unused on each sp hybridized atom, and these overlap to give two pi-bonds following the formation of a sigma bond (a triple bond), as shown below.