Receptor Terminology; Law of Mass Action
Brief Historical Background
Some terms and definitions
- Affinity: the "tenacity" by which a drugs binds to
its receptor.
- Discussion: the solution of a lipid containing drug in the bilayer
may be essentially irreversible: is this a high affinity event?
- Intrinsic activity (=efficacy?): the relative
maximal response caused by a drug in a tissue preparation. A full agonist causes a maximal effect equal to that of
the endogenous ligand (or sometimes another reference compound if the endogenous ligand is not known); a partial agonist
causes less than a maximal response.
- Intrinsic efficacy: a drug’s ability to affect
a receptor and cause a biological response (hence a property of a drug). (Discussion: can a drug have "negative efficacy"?).
- Potency: broad definition: wide the ability of a drug
to cause a measured biological change; narrow definition: wide the ability of a drug to cause a measured functional change.
The pharmacologist’s dream:
From a pharmacological perspective, one might simplify drug, hormone,
or neurotransmitter action via the following scheme:

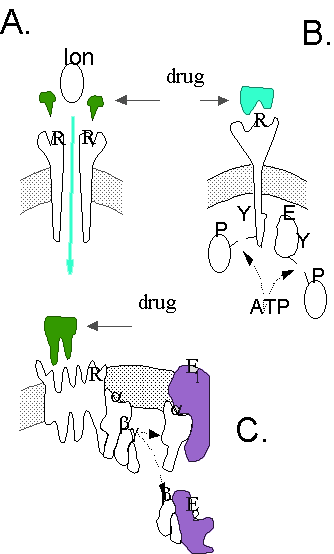
Ideally, we would wish to understand, and then predict, all of the
properties of how a drug can cause a response in any tissue. Unfortunately, there are many factors that make achieving this
goal at best difficult, and at worst, impossible. For example, events that affect the equilibrium of the drug at the receptor
(limited diffusion on a macro or micro scale, metabolism, entrapment, etc.) can cause experimental results to deviate from
theory. Even more importantly, the production of a stimulus often does not have a one-to-one correspondence to the measured
response. The response caused by an activated receptor can involve a variety of different mechanisms (see cartoon in Figure
1). Some receptors directly effect the response of interest (e.g., the ionotropic receptor in A.). Even in this case,
other factors (including allosteric modulators, cofactors, etc.) that can influence the observed response. In other cases,
like a tyrosine kinase (B.), the receptor may itself be modified (e.g., phosphorylated) in the process of catalyzing
a reaction (phosphorylation in this example). In the case of G protein coupled receptors (C.), the receptor may start
a cascade of biochemical events due to actions at several effectors, and in some cases, also may be phosphorylated itself.
Theories of Drug-Receptor Interaction
There have been several major theories that have been proposed to
provide a theoretical basis for understanding, modeling, and thereby predicting, drug response. Three of the most widely known
of these schemes are described as follows:
- Occupation Theory: the idea that a response emanates
from a receptor only when it is occupied by an appropriate ligand (drug).
- Rate Theory: the idea that a response emanates from
a receptor in proportion to the kinetic rate of onset and offset of drug binding to the receptor.
- Operational Model: a modified, semi-empirical approach
that is based on occupancy theory modified to incorporate a factor relating agonist-receptor complex and response.
All of these theories have specific strengths and appeal, and all
have significant failings. In general, rate theory is now considered to be the one of least utility. Conversely, since the
advent of radioreceptor methods in the mid-1970’s, it is now possible to measure drug-receptor interactions directly,
making occupation theory of particular interest because receptor occupation can be measured directly for the first time. As
molecular tools begin to provide ways of studying several sequential molecular events, operational theory or direct multi-step
models can certainly be applied.
Law of Mass Action
Although there are a plethora of complexities that arise, it is
true that the large majority of experiments [especially those using radioreceptor methods (radioligand binding methods)] are
based on very simple application of the law of mass action. In the case of a drug (ligand) interacting with a homogeneous
population of receptors, this relationship can be expressed:
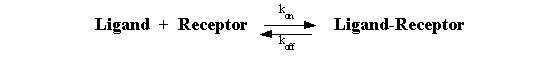
Binding occurs when ligand and receptor collide (due to diffusion)
in the correct orientation and with enough energy. The rate of association (number of binding events per unit of time) equals
[Ligand]*[Receptor]*kon.
Once binding has occurred, the ligand and receptor remain bound
together for a random amount of time. The rate of dissociation (number of dissociation events per unit time) equals [ligand*receptor]*koff.
The probability of dissociation is the same at every instant of time. The receptor doesn't "know" how long it has been bound
to the ligand. After dissociation, the ligand and receptor are the same as at they were before binding. If either the ligand
or receptor are chemically modified, then the binding does not follow the law of mass action.
Equilibrium is reached when the rate of formation of new ligand-receptor
complexes equals the rate at which existing ligand*receptor complexes dissociate. By definition, at equilibrium this means
that:
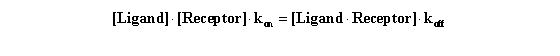
Rearrange that equation to define the equilibrium dissociation constant
KD.
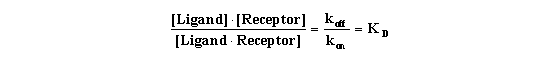
This equation gives you a feel for what KD means. When
the ligand occupies half the receptors, the concentration of unoccupied receptors equals the concentration of occupied receptors:
[Receptor] = [Ligand*Receptor].This can only be true when KD equals [Ligand]. In other words, the KD
is the concentration of ligand that, at equilibrium, will cause binding to half the receptors.
 |
REMEMBER, the KD is the equilibrium dissociation
constant, whereas the koff is the dissociation rate constant. The two measure different things and are expressed
in different units. |
| Variable |
Name |
Units |
| kon |
Association rate constant or "on" rate constant |
M-1min-1 |
| koff |
Dissociation rate constant or "off" rate constant |
min-1 |
| KD |
Equilibrium dissociation constant |
M |
| KA |
Equilibrium association constant (=1/KD) |
M-1 |
A term that is sometimes useful to pharmacologists is fractional
occupancy. Based on the law of mass action, this term describes receptor occupancy at equilibrium as a function of
ligand concentration. Specifically:
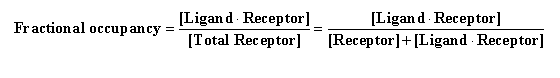
From the equation for KD derived above, it is seen that:
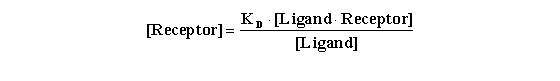
One can substitute this value for [Receptor] in the denominator
of the equation for fractional occupancy and after simplifying, obtain the following (Do this yourself to see if it’s
correct):
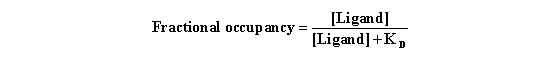
This equation assumes equilibrium. To make sense of it, think about
a few different values for [Ligand]. When [Ligand]=0, the occupancy equals zero. When [Ligand] is very high (many times KD)
, the fractional occupancy approaches 100%. When [Ligand]=KD, fractional occupancy is 50%.
Although termed a "law", the law of mass action is simply a model
that can be used to explain some experimental data. As noted above, this model is not useful in all situations.
- The model assumes that all receptors are equally accessible to
ligands.
- The model ignores any states of partial binding. According to the
model, receptors are either free or bound to ligand. It also doesn’t allow for more than one affinity state (although
this can be accommodated in some ways).
- The model assumes that the ligand is not altered by binding.
- The model assumes that binding is reversible.

|
Just because binding is constant over time does not mean the
system is in equilibrium. Other reactions could be happening as well, especially when agonists are used. Many investigators
use the term "steady state" to describe binding that has plateaued, and reserve the term "equilibrium" to describe the ideal
model. |

The Theoretical Basis for Characterizing Receptors using Saturation
Radioligand Assays
Radioreceptor assays were first developed in the early 1970’s.
They were based on two very simple, but very elegant concepts.
- If a ligand had high affinity for a macromolecular target (as had
been shown by classical pharmacological studies over many decades), it should be thermodynamically possible to measure the
binding of the ligand to the receptor without the need to perform equilibrium dialysis (the only method then used) as long
as one could separate the ligand-receptor complex from the free ligand.
- By labeling ligands with appropriate radioactive atoms, one could
detect the ligand-receptor sensitively and rapidly. (This was the key point, since chemical methods were neither sufficiently
sensitive nor inexpensive for this use.)
The sections that follow will discuss many of the actual considerations
for such assays, but it is first important to understand the conceptual basis for this approach. We begin with the simple
law of mass action derived earlier:
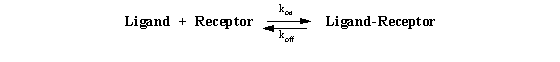
which led to the following equation:
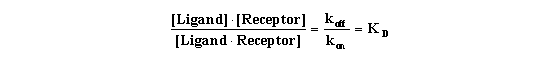
The receptor that has radioligand bound to it is called, in lab
jargon, "Bound" or "B". The ligand that is not bound to receptor is called "Free" or "F". Both
of these are measurable experimentally, as will be discussed below. This allows us to substitute these more common terms in
the previous equation.
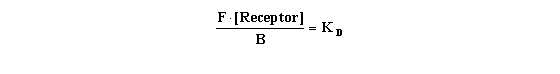
Since F and B are independent variables, and we wish
to solve for KD, it is necessary to be able to quantify the unbound receptor. This is technically impossible at
present. Yet we know that:
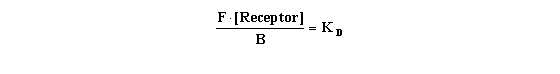
In fact, the total amount of receptor present (i.e., the maximal
number of binding sites) is termed "Bmax" in lab jargon, and is another desired experimental parameter.
Thus, with more common lab terms:

or by rearranging:

If we take the equation from above:
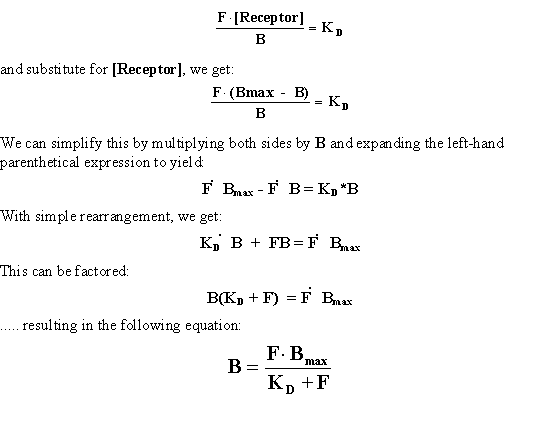
This equation should make clear the experimental design. One has
an independent variable (F) and a dependent variable (B), and a successful experiment should allow one to arrive
at estimates of two biologically meaningful constants: KD and Bmax. Hopefully this equation
will look somewhat familiar, as it is functionally identical to the Michaelis-Menten equation of enzyme kinetics.
